The Ability of Carbon to Attract Electrons Is
By sharing outershell electrons carbon and hydrogen their valence and become more stable. 30 The ability of an atom in a molecule to attract electrons is best quantified by the__________.

Solubility Easy Science Solubility Easy Science Science Flashcards
Which of the following statements about water is CORRECT.

. An atom or a functional group can have this ability and some have stronger electronegativity than. Denis23 38 1 year ago. Water is a polar molecule c.
Corr auonship between electroneoativity an atomic fòr the elements of Period 3. Carbon atoms attract electrons from other atoms. Greater than that of nitrogen and oxygen D.
Oxygen fluorine C nitrogen D chlorine The ability of carbon to attract electrons is greater than that of nitrogen but less than that of. The ability of atoms to attract electrons is referred to as. Electronegativity is a measure of an atoms ability to attract the shared electrons of a covalent bond to itself.
A electronegativity b electron charge-to-mass ratio c diamagnetism d first ionization potential e paramagnetism. A van der Waals attraction. Electronegativity is the relative ability of an atom involved in covalent bonding to pull electrons toward itself.
Purest native form of uncombined carbon. It is the ability of an atom in a molecule to attract electrons in itself. The ability of carbon to attract electrons is less than that of nitrogen and oxygen as the elements Li and F in period 2 of the periodic table are considered in succession how do the relative electronegativity and the covalent radius of each successive element compare.
Click hereto get an answer to your question Fill in the blanks with the appropriate option. A measure and or tendency for the ability to attract electrons in a chemical bond is the atoms electronegativity. Experts are tested by Chegg as specialists in their subject area.
If atoms bonded together have the same electronegativity the shared electrons will be equally shared. The ability of atoms to attract electrons is referred to as. Greek letter chi χ pronounced ky as in sky which is defined as the relative ability of an atom to attract electrons to itself in a chemical compound.
The ability of an atom to attract shared electrons to itself is called i. Fluorine is assinged the oxidation number of -1 because it attracts the electrons in the bond more strongly than the carbon does. Which of the following species has the highest polarizability.
Question 1 1 point The ability of a carbon atom in a bond to attract electrons is greater than that of nitrogen but less than that of oxygen. Van der Waals attraction e. It is the ability of an atom to attract electrons in chemical bonding.
The ability to attract electrons is called electronegativity. Carbon has four valence electrons in its shell carbon can share electrons with up to four different atoms. Water has good solvent properties b.
Greater than that of nitrogen but less than that of oxygen B. Water is the most abundant molecule in living cells d. It generally iv across a period and v down a group.
The ability to attract electrons. Earlier we said the fluorine in CF 4 has and oxidation number of -1. Carbon is an element with a known electronegativity value 255 on the Pauling.
Less than that of nitrogen but greater than that of oxygen C. N to other elements. Carbon has four valence electrons has one valence electron.
This sharing allows each atom achieve its of electrons greater stability. We review their content and use your feedback to keep the quality high. The ability of carbon to form four covalent bonds c the ability of those bonds to rotate freely d All of these choices are correct.
Carbon atoms have strong attractio. Greater than that of. A paramagnetism B diamagnetismC electronegativity D electron change-to-mass ratio E first ionization potential Answer.
Electrons in a chemical bond. The carbon-oxygen double bond in formaldehyde methane and the carbon-nitrogen triple bond in acetonitrile cyanomethane are both polar. B C D 28.
NOTA Measures how strongly the atom attracts ADDITIONAL electrons Measures how strongly the atom holds to its electrons. Why is the bond angle in a water molecule less than the bond angle of methane. - 11748841 baylonjas13 baylonjas13 02032021 Science Junior High School answered.
The electronegativity of an element is a measure of the elements ability to attract the electrons which are in a bond. The central oxygen atom in water has two lone pairs of electrons whereas the central carbon atom in methane has no lone pairs. Less than that of nitrogen but greater than that of oxygen.
B C D Atomic Number Atomic Number Atomic Number Atomic Number A B 27. The ability of an atom in a molecule to attract. Covalent bonds when share electrons.
C Carbon atoms can form many types of bonds with other carbon. The ability of an atom in a molecule to attract electrons is best quantified by the __________. It is generally measured on the ii scale.
The ability of carbon to attract electrons is A. An arbitrary value of iii is assigned to fluorine have greatest ability to attract electrons. Less than that of nitrogen and oxygen 10.
The central hydrogen atom in water has one lone pair of electrons whereas the central carbon atom in methane has two lone pairs. Methane CH 4 organic contains covalent bonds.

File Electron Shell 050 Tin Svg Wikimedia Commons Electron Affinity Science Chemistry Electrons

Nonpolar Covalent Bond Covalent Bonding Chemistry Lessons Chemistry Basics

Coordination Compound Ligand Field And Molecular Orbital Theories Britannica

Magnetic Fields Can Control Heat And Sound Magnetic Field Online Dating Apps Album Songs

Theorganisedstudent Study Notes Notes Inspiration Chemistry Notes

Co Insertion Chemistry Britannica

Carbon Group Element Chemical Elements Britannica

Osmotic Pressure Easy Science Osmotic Pressure 10th Grade Science Study Skills

Electronegativity Physics Britannica
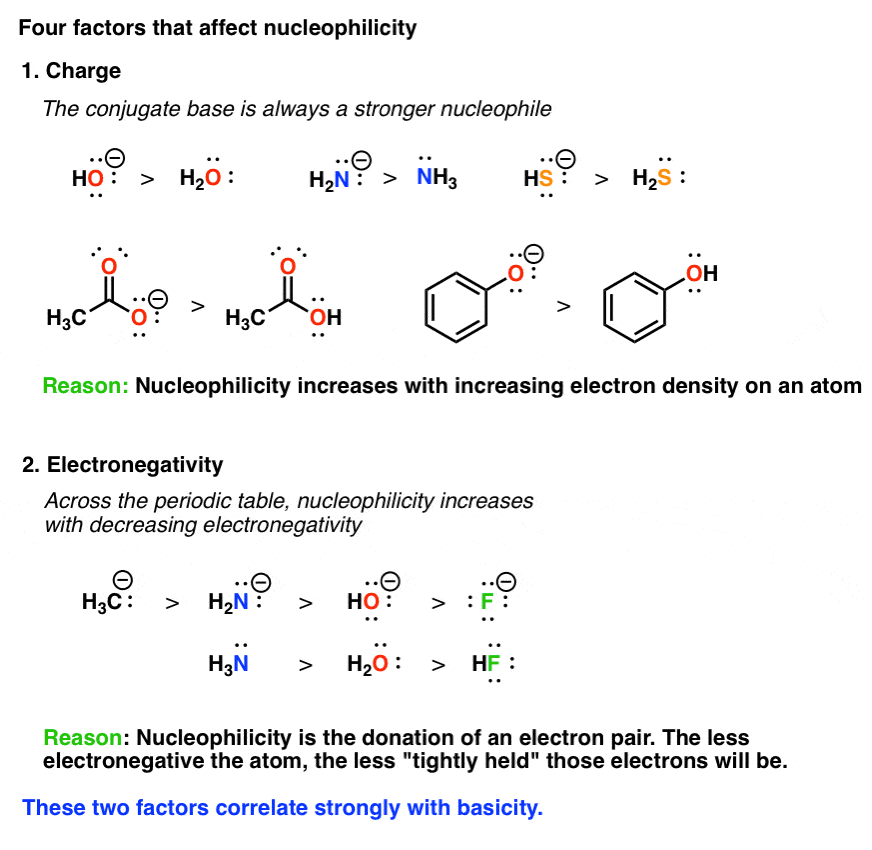
What Makes A Good Nucleophile Master Organic Chemistry

Electron Diagram Of Magnesium Electron Dot Structure For Electron Configuration Electrons High School Chemistry

Chemistry Unit 3 Periodicity Ppt Download

Graphene Makes Light Work Of Optical Signals How To Make Light Nanotechnology Thermoelectric Materials

Crystal Types Of Bonds Britannica

Electronegativity Which Factors Influence Electronegativity Chemistry Chemistry Science Chemistry Covalent Bonding

Definition And Classification Of Chemical Compounds Britannica
Ppt Chemical Bonds Powerpoint Presentation Free Download Id 2962570

Comments
Post a Comment